Solutions for Clinical Trial Drugs
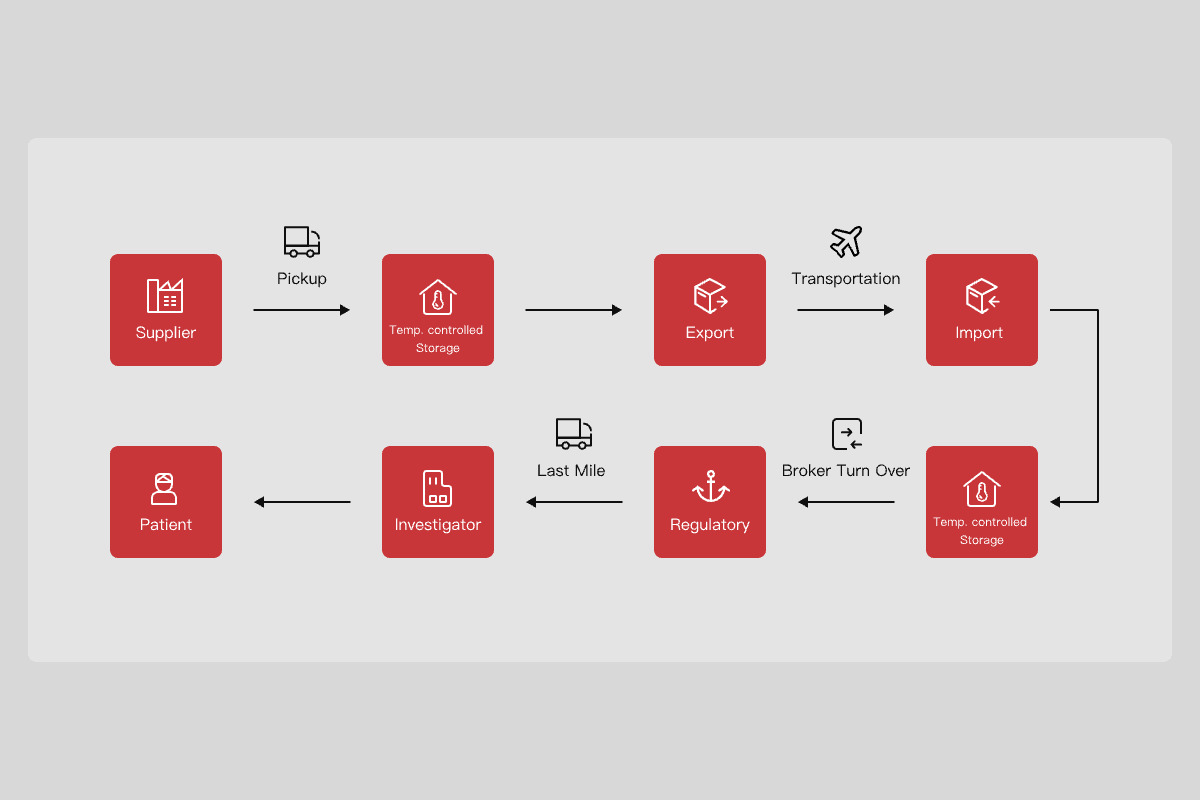
In response to the unique demands of the clinical trial drug supply chain, SF Supply Chain at Shanghai Zhangjiang Yaogu has built a dedicated pharmaceutical warehouse for customers conducting pharmaceutical clinical trials. The pharmaceutical warehouse has received dual certification from the World Health Organization (WHO) for Good Manufacturing Practice (GMP) and Good Distribution Practice (GDP). We are equipped to provide a variety of services to global and local pharmaceutical research and development companies. These services cover the clinical trial supply chains and design of drug packaging solutions, cold chain transportation routes and the design of temperature-controlled packaging solutions for drugs, the storage and distribution of drugs requiring different temperatures, direct delivery and pickup services to and from patients' homes(DTP&DFP), and drug recycling services. We also manage the clinical trial supply chain for cell and gene therapies, providing liquid nitrogen transportation services via dedicated couriers or vehicles. Simultaneously, leveraging our network of 29 GMP-certified pharmaceutical warehouses for clinical trials globally, SF Supply Chain assists innovative pharmaceutical companies in expanding overseas, conducting global clinical trials, and commercialization of drugs
One-stop Clinical Trial Supply Chain Services to the Pharmaceutical Research & Development sector, optimizing the management of the Clinical Trial Drug Supply Chain
Customized Solutions according to Different Projects to Accelerate the Clinical Trial Process and effectively Reduce Related Logistics Expenditures
GMP-compliant Drug Packaging and Labeling
Professional Warehouse Management System (WMS) and Order Management System
GDP-compliant Temperature-controlled Transportation Solutions
Direct-to-Patient (DTP) Delivery and Direct-from-Patient (DFP) Pickup Services
*DTP & DFP apply to clinical trial drug distribution services
Raw material pharmaceutical factory, pharmaceutical factory, laboratory, CMO enterprise, R&D pharmaceutical manufacturer, clinical project test center, Cell and Gene Therapy, GMP factory




